Vibrant® Prescribing Guidance For Clinicians
Prescribing Vibrant® in Your EHR
Select Vibrant in your EHR
* New patients – select code 60009-0189-82
* Refills – select code 60009-0189-80
Please make sure you include in the prescription notes the patient’s ICD-10 diagnosis code(s) as well as any previous use of prescribed constipation medications.
Pharmacy
Use of our partner Specialty Pharmacy is required, but it's easy.
Pharmacy for Prescriptions sent before May 1, 2025:
How to choose Carepoint in your EHR
* Search by zip code – 60173
* Verify address prior to sending prescription – 9 E. Commerce Drive, Schaumburg, IL
* Add to favorites for convenience
* Please note that Vibrant is available only via Carepoint™ specialty pharmacy. You can also fax the prescription to (855) 237-9113.
Pharmacy for Prescriptions sent as of May 1, 2025:
Effective May 1, 2025
Announcing Our New Specialty Pharmacy Partnership
Phone: 866-359-7532
Fax: 866-847-7049
NPI: 1548264591
Prior to May 1st, 2025, please continue to use CarePoint™.

Effective May 1, 2025:
All prescriptions should be sent to EVERSANA, our new Specialty Pharmacy Partnership.
Phone: 866-359-7532
Fax: 866-847-7049
NPI: 1548264591
*Process all prescriptions through your EHR by choosing EVERSANA.
Dear Patients and Healthcare Providers,
We are excited to announce that the Vibrant System will soon be available through a new specialty pharmacy provider, EVERSANA, as part of our ongoing commitment to ensuring seamless access and exceptional service for all patients and providers.
This transition start date is effective May 1, 2025.
New Specialty Pharmacy Provider Contact Information:
*Process all prescriptions through your EHR by choosing EVERSANA.
EVERSANA Specialty Pharmacy (Integrated with the VibrantConnect Team)
Phone: 866-359-7532
Fax: 866-847-7049
NPI: 1548264591
For prescription transfers, new orders, or any assistance with the Vibrant System, please contact our VibrantConnect team directly using the details above. You will have the option to choose from interactive voice response to guide to the appropriate team to support your questions. For product related, Medical / Clinical, and or technical support continue to reach out to our VibrantConnect team…
Phone: 866-359-7532
Email: service@vibrantgastro.com
We appreciate your continued trust in us and look forward to working with EVERSANA to provide the highest level of support to our patients and healthcare providers. Should you have any questions or need further assistance, please don’t hesitate to reach out.
Best regards,
Asaf Bar
CEO, Vibrant Gastro
Additional Prescribing & Indication Guidance
Vibrant is indicated for the treatment of adults with chronic idiopathic constipation who have not experienced relief of their bowel symptoms by using laxative therapies at the recommended dosage for at least 1 month.
| Product Name | Vibrant Starter Kit |
|---|---|
| NDC | 60009-0189-82 |
| Indication | Treatment of chronic idiopathic constipation |
| Dosage Form | Capsule |
| Dispense Quantity | 1 |
| Dispense Unit | 1 ea Box |
| Number of Supply in One Box | 1 Pod, 20 Capsules |
| Days Supply | 30 days |
| SIG | As directed by a physician. Orally ingested up to 5 times per week. The Capsules should be taken once daily, on any of the 5 days out of the week, shortly before going to sleep. |
| Notes to Pharmacist | Relevant ICD-10 Code for CIC and examples of previous medications tried and failed for treatment may streamline Prior Authorization Process |
| Specialty Pharmacy | Carepoint Pharmacy 9 E. Commerce Drive Schaumburg, IL 60173 (855) 237-9112 |
Example Dosing Screenshot:

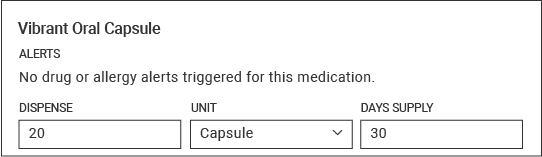
| Product Name | Vibrant Capsule (Refill) |
|---|---|
| NDC | 60009-0189-80 |
| Indication | Treatment of chronic idiopathic constipation |
| Dosage Form | Capsule |
| Dispense Quantity | 20 |
| Dispense Unit | Capsule |
| Number of Supply in One Box | 20 Capsules |
| Days Supply | 30 days |
| SIG | As directed by a physician. Orally ingested up to 5 times per week. The Capsules should be taken once daily, on any of the 5 days out of the week, shortly before going to sleep. |
| Notes to Pharmacist | Relevant ICD-10 Code for CIC and examples of previous medications tried and failed for treatment may streamline Prior Authorization Process |
| Specialty Pharmacy | Carepoint Pharmacy 9 E. Commerce Drive Schaumburg, IL 60173 (855) 237-9112 |
Example Dosing Screenshot:
Need Help?
If you can’t find Vibrant in your EHR or have questions, email us at help@vibrantgastro.com
Vibrant should not be used if your patient
- has a history of complicated/obstructive diverticular disease.
- has a history of intestinal or colonic obstruction, or suspected intestinal obstruction.
- currently has clinical evidence of significant gastroparesis.
- has a history of significant gastrointestinal disorder, including any form of inflammatory bowel disease or gastrointestinal malignancy (celiac disease is excepted if the subject has been treated and is in remission), and/or anal fissures and fistulas.
- has a history of Zenker’s diverticulum, dysphagia, esophageal stricture, eosinophilic esophagitis, or achalasia.
- is pregnant or lactating.
Adverse events/side effects:
Potential adverse events associated with the use of this device may include abdominal pain, abdominal distension, abdominal discomfort, vomiting, nausea, blood in stool, diarrhea, flatulence, and proctalgia. In rare cases, obstruction may occur.
Note that the safety and effectiveness of the Vibrant System for long-term use in the indicated population, i.e., for more than 8 weeks, has not been evaluated.
Warnings:
- The Vibrant Capsule is MR unsafe. The device has not been evaluated for safety and compatibility in the MR (magnetic resonance) environment. It has not been tested for heating, migration, or image artifact in the MR (magnetic resonance) environment. If patient requires an MRI, verify Capsule expulsion via abdominal X-ray before undergoing an MRI examination.
- The Capsule should be kept away from implants such as pacemakers, defibrillators, nerve stimulators, and other devices that could be affected by proximity to a DC (direct current) magnetic field.
- Vibrant Capsules must be stored in a safe place, out of the reach of children and/or infants.
- If a child has accidentally swallowed an unused Vibrant Capsule, he/she should be brought immediately to a hospital.

Contact us about patient options for participating in our post-market clinical trial.
Receive a quick online training video on Vibrant.
The training is only 7 minutes long, covering information about Vibrant, phase 3 data, and how to prescribe to suitable patients.
