Explore Vibrant®: A Transformative Approach to Chronic Constipation* (CIC)
Vibrant® is clinically proven to deliver effective relief with minimal side effects—only 1.2% of patients report diarrhea.* For clinicians seeking to treat Chronic Idiopathic Constipation (CIC), Vibrant® offers a safer, more predictable outcome for many patients.
Prescribing Vibrant®: Specialty Pharmacy Information & EHR Guidance
Effective May 1, 2025
Announcing Our New Specialty Pharmacy Partnership
Phone: 866-359-7532
Fax: 866-847-7049
NPI: 1548264591
Prior to May 1st, 2025, please continue to use CarePoint™.

Vibrant® is Safe & Effective for Chronic Idiopathic Constipation
Vibrant is indicated for the treatment of adults with chronic idiopathic constipation who have not experienced relief of their bowel symptoms by using laxative therapies at the recommended dosage for at least 1 month.
The highly effective mechanism of action stimulates the colon’s natural rhythm
In patients with chronic idiopathic constipation (CIC), colon activity is often out of sync.
Vibrant is a capsule that, when activated and swallowed, sends out pulsed micro-vibrations to stimulate the colon locally, which induces peristalsis and helps the colon contract mechanically, synchronizing with its biological clock.2
Proven effective with an excellent safety profile
Vibrant is a novel, non-pharmacological, intraluminal therapy that is proven to be efficacious and safe in patients with chronic idiopathic constipation (CIC).2
It is indicated for the treatment of adults with chronic idiopathic constipation who have not experienced relief of their bowel symptoms by using laxative therapies at the recommended dosage for at least 1 month.
Clinical Data
Randomized, double-blind, multi-center, placebo-controlled phase 3 clinical trial with 312 adult patients diagnosed with chronic idiopathic constipation.
The primary efficacy endpoints were an increase of 1 or more complete spontaneous bowel movements per week (CSBM responder) or 2 or more CSBMs per week (CSBM ) from baseline during at least 6 of the 8 weeks.
Significant improvements across all efficacy measures
Primary Endpoints
p=0.001
p=0.008
Treatment Arm
Vibrant
Placebo
- Vibrant Capsule led to one additional bowel movement in approximately 40% of patients compared with 23% of people who were in the control arm.2
- Approximately 23% of people taking Vibrant had two additional bowel movements per week, compared with approximately 12% of people taking placebo.2
Secondary Endpoints
constipation symptoms
Straining Score
0.0126
Stool Consistency
<0.0001
=0.002
Constipation symptoms
Straining Score
0.0126
Stool Consistency
<0.0001
=0.002
Treatment Arm
Vibrant
Placebo
Vibrant demonstrated significant improvement in
- stool frequency, which was seen as early as week 1 and continued throughout the study.
- other bowel and abdominal symptoms and quality of life.
The regimen established tolerability with no significant adverse events.
Vibrant provides a novel, non-pharmacological, intraluminal therapy that is effective, safe, and well tolerated in patients with CIC.2
Vibrant is available only via Carepoint Speciality Pharmacy. Lookup via EHR or fax the prescription to (855)237-9113.
| Product Name | Vibrant Starter Kit |
|---|---|
| NDC | 60009-0189-82 |
| Indication | Treatment of chronic idiopathic constipation |
| Dosage Form | Capsule |
| Dispense Quantity | 1 |
| Dispense Unit | 1 ea Box |
| Number of Supply in One Box | 1 Pod, 20 Capsules |
| Days Supply | 30 days |
| SIG | As directed by a physician. Orally ingested up to 5 times per week. The Capsules should be taken once daily, on any of the 5 days out of the week, shortly before going to sleep. |
| Notes to Pharmacist | Relevant ICD-10 Code for CIC and examples of previous medications tried and failed for treatment may streamline Prior Authorization Process |
| Specialty Pharmacy | Carepoint Pharmacy 9 E. Commerce Drive Schaumburg, IL 60173 (855) 237-9112 |
Example Dosing Screenshot:

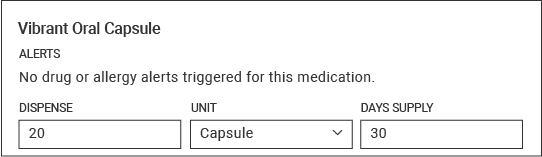
| Product Name | Vibrant Capsule (Refill) |
|---|---|
| NDC | 60009-0189-80 |
| Indication | Treatment of chronic idiopathic constipation |
| Dosage Form | Capsule |
| Dispense Quantity | 20 |
| Dispense Unit | Capsule |
| Number of Supply in One Box | 20 Capsules |
| Days Supply | 30 days |
| SIG | As directed by a physician. Orally ingested up to 5 times per week. The Capsules should be taken once daily, on any of the 5 days out of the week, shortly before going to sleep. |
| Notes to Pharmacist | Relevant ICD-10 Code for CIC and examples of previous medications tried and failed for treatment may streamline Prior Authorization Process |
| Specialty Pharmacy | Carepoint Pharmacy 9 E. Commerce Drive Schaumburg, IL 60173 (855) 237-9112 |
Example Dosing Screenshot:
Vibrant should not be used if your patient
- has a history of complicated/obstructive diverticular disease.
- has a history of intestinal or colonic obstruction, or suspected intestinal obstruction.
- currently has clinical evidence of significant gastroparesis.
- has a history of significant gastrointestinal disorder, including any form of inflammatory bowel disease or gastrointestinal malignancy (celiac disease is excepted if the subject has been treated and is in remission), and/or anal fissures and fistulas.
- has a history of Zenker’s diverticulum, dysphagia, esophageal stricture, eosinophilic esophagitis, or achalasia.
- is pregnant or lactating.
Adverse events/side effects:
Potential adverse events associated with the use of this device may include abdominal pain, abdominal distension, abdominal discomfort, vomiting, nausea, blood in stool, diarrhea, flatulence, and proctalgia. In rare cases, obstruction may occur.
Note that the safety and effectiveness of the Vibrant System for long-term use in the indicated population, i.e., for more than 8 weeks, has not been evaluated.
Warnings:
- The Vibrant Capsule is MR unsafe. The device has not been evaluated for safety and compatibility in the MR (magnetic resonance) environment. It has not been tested for heating, migration, or image artifact in the MR (magnetic resonance) environment. If patient requires an MRI, verify Capsule expulsion via abdominal X-ray before undergoing an MRI examination.
- The Capsule should be kept away from implants such as pacemakers, defibrillators, nerve stimulators, and other devices that could be affected by proximity to a DC (direct current) magnetic field.
- Vibrant Capsules must be stored in a safe place, out of the reach of children and/or infants.
- If a child has accidentally swallowed an unused Vibrant Capsule, he/she should be brought immediately to a hospital.
- The Vibrant Capsule is magnetic resonance (MR) unsafe. If an MRI is required, verify that no Capsules are currently in your body via abdominal X-ray before undergoing an MRI examination.
If you can’t find Vibrant in your EHR or have questions, email us at help@vibrantgastro.com
3 simple steps to relief with Vibrant
1. Activate
The patient places the Capsule in the Pod for an automatic activation.
2. Ingest
The patient swallows the activated Capsule with a full 8 oz. glass of water.
3. Monitor
For the best experience, encourage patients to monitor their treatment using the free Vibrant app, downloadable from any app store.